|
Dr Tom Cromarty Editor Interests: Paediatric Emergency Medicine, Medical Engagement and Leadership, Simulation, Quality Improvement, Research Twitter: @Tomcromarty |
Welsh Research and Education Network
WREN BlogHot topics in research and medical education, in Wales and beyond
Dr Celyn Kenny Editor Interests: Neonates, Neurodevelopment, Sepsis, Media and Broadcasting Twitter: @Celynkenny |
|
Guest Blogger Dr Jordan Evans N Engl J Med 2018;378:2275-87. DOI: 10.1056/NEJMoa1716816 If there’s one thing we all know about manging diabetic ketoacidosis (DKA), it’s the importance of being extremely cautious with fluid management due to the risk of causing iatrogenic cerebral oedema right?...Wrong! Once again, like John Snow, we unfortunately ‘know nothing!’. When will the childhood lies end, Father Christmas isn’t real, the Easter bunny’s not real and now this, the most painful blow yet. In this PECARN (Pediatric Emergency Care Applied Research Network) study, published in the New England Journal of Medicine, Nathan Kuppermann et al investigated the influence of intravenous fluid administered on the rates of neurological injury in children with DKA. What was the reason for the study? Brain injury occurs in the region of 0.5% - 1% of DKA presentations. It presents with sudden neurological deterioration. Patients without a marked neurological deterioration may have more mild neurological impairments e.g. memory / cognitive impairment. As all of us were likely taught, brain injury in DKA has long been thought to be iatrogenic, secondary to fluid administration causing cerebral oedema due to osmotic gradients. The evidence for this school of thought is however lacking and evidence has emerged actively disputing it. Alternative explanations have been proposed that there may be something about being particularly unwell with DKA that leads to the neurological injury, possibly inflammatory or vascular changes. The more unwell the patient is the more likely they are to require fluid resuscitation and therefore an association between severe DKA with brain injury and fluid administration could be mistaken as causation. Remember that association is not proof of causation! Oedema may develop secondary to the brain injury itself as it does in other mechanisms of injury such as trauma. Where did the study take place? The study was conducted across 13 centres in the United States. Who did they include ? Children aged 0 – 18 years with a diagnosis of DKA (pH < 7.25, glucose > 16.7 mmol/L. Children with GCS <12 were excluded two years into study (due to concerns of treating clinicians). What did the investigators do? Children presenting to study centres with DKA were randomly assigned to one of the following four groups; 1) 0.9% saline fast administration 2) 0.9% saline slow administration 3) 0.45% saline fast administration 4) 0.45% saline slow administration The fast group were given a 20ml/kg bolus which was then followed with replacement of a 10% fluid deficit with the first half of the deficit volume being replaced over 12hrs and the rest over the next 24 hrs. The slow group were given a 10ml/kg bolus which was then followed by replacement volume for a 5% deficit which was given over 48hrs. The primary outcome was a decline in mental status (GCS <15 on two occasions within first 24 hours of treatment. Patients and parents were blinded but not the clinician. Secondary outcomes; clinically apparent brain injury (neurological deterioration leading to clinical decision to treat for raised ICP, intubation or death), short term memory, memory & IQ at 2 months and 6 months. What were the results? 4912 patients met the inclusion criteria. Due to the complexities of the study of these there were a total of 1389 episodes of DKA included in the study (from 1255 different children). There was a fall in GCS in 3.5% of these epsiodes (48 episodes). There was clinically apparent brain injury in 0.9% (12 episodes). There was no difference between the two groups for any of the outcomes. (Some of the raw results favoured the fast fluid administration group although none of these results reached a statistically significant level). Conclusions Kupperman et al. rightly concluded that ‘Neither the rate of administration nor the sodium chloride content of intravenous fluids significantly influenced neurologic outcomes in children with diabetic ketoacidosis’. Take home message for practice in Wales 1. We no longer need to be as anxious about giving fluid too fast in DKA
2. If you need to give a bolus for shock do so without fear of causing cerebral oedema, this study provides evidence that it’s not harmful. 3. With regards to the fluid deficit replacement although this study shows we could safely give it a little faster stick to current practice of the Wales DKA protocol as it doesn’t show any improvement in outcomes for the faster administration of IV fluids. 4. It’s worth remembering that there is good evidence for using 0.9% saline for maintenance IV fluids in children (to prevent hyponatraemia) so it’s not clear why they chose to have arms in this trial with both 0.9% saline and 0.45% saline.
1 Comment
Dr Annabel Greenwood (ST3), inspired by Dr Ian Morris (Consultant Neonatologist, UHW)
Relevance– How important is the content of the article to me, my patients, and my practice? The title and the abstract will help you answer these questions. Internal validity– How sound is the study? i.e. how accurate is the study? Look at the methods to help ascertain this. External validity– How applicableis the article to my practice? i.e how much can I generalize it? The methods and results sections will help you assess the external validity. Now, with those 3 key principles in mind, let us picture the scene… You’re an ST7 trainee, approaching the end of your paediatric training, years of hard work and dedication behind you, your CCT within touching distance. You’re at your START assessment, the last hurdle before consultancy, and now for your critical appraisal station. Twenty minutesto read the article AND then appraise?! How on earth am I going to do this I hear you cry?! Stay calm, take a deep breath, and burst forth the 9 key questions that will help you ace the station! Describe the study What is the type of study (principal method) What type of question is being asked? (e.g. Diagnostic? Therapeutic? Economic?) Where was the study carried out? (is it multicentred or single study) What are the key features?
Describe the research question
What is the relevance of the question? This is usually described in the introduction. Is it an important question? Is it worth reading on? Does it correlate to your clinical practice/scenario? Describe the methods Use the PICO template, but expand…. What are the inclusion/exclusion criteria? Is there any masking or blinding? Are there any secondary outcomes? Comment on the internal validity i.e. how accurate is the study? Use various checklists e.g. CONSORT, STROBE, PRISMA Is there a risk of bias? Summarise the primary results Summarise the key secondary results(briefly) Comment on the External validity– i.e. describe the generalizability of these findings Were the inclusion/exclusion criteria reflective? Limitations? Consider the paper in conjunction with other studies i.e. how does it fit with the evidence out there? Conclusion Overall, does this paper help you answer your clinical question? Important to look at the clinical context! ….And just like that, you did it! 9 logical, concise steps to critical appraisal for the START assessment. Of course, this is only a suggested approach, but one which I will most certainly be adopting for future critical appraisals. Now, where did I put that journal?.....
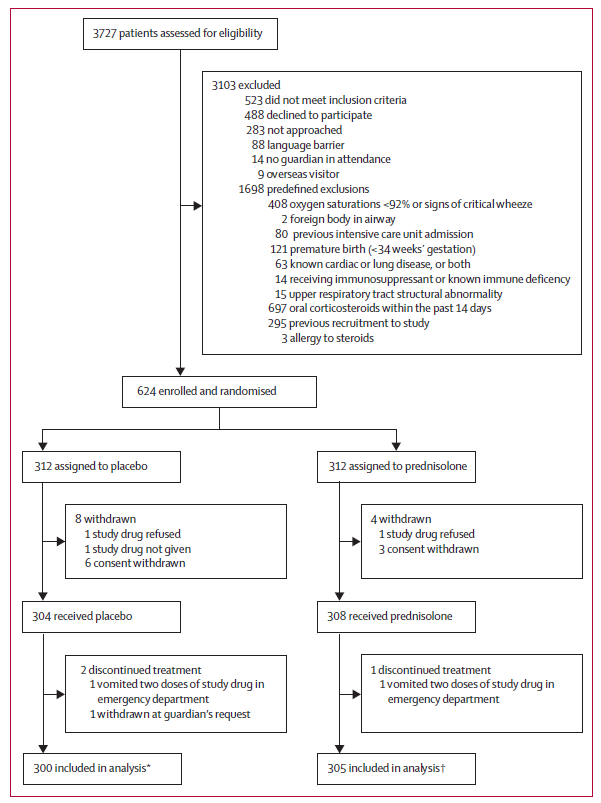
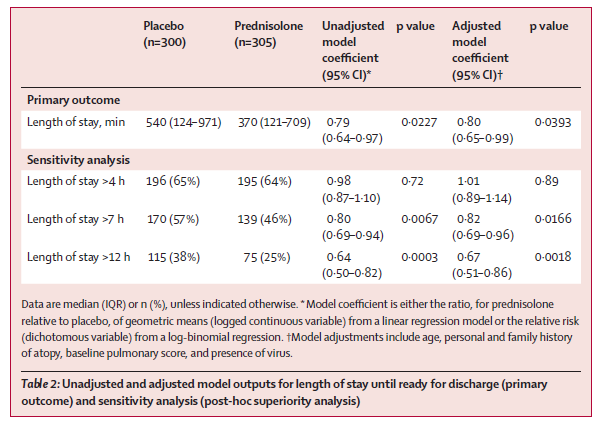
Rebecca Broomfield With a busy family and work life my pile of “must read” journal articles grows larger and larger month by month. However, when the article below popped up on my twitter feed during January it was so relevant to what I do currently every shift and there are a significant number of differing views and options it immediately jumped to the top of the list! (And I actually read it!) The topic of whether to give steroids or not to give steroid when a preschool child presents with wheeze is one which causes huge debate and which I frequently question my own management on. A previous paper published by Panickar et al in 2009 changed our practise and guidelines, despite having questions about the applicability of the study. However, the paper outlined below suggests that we still have more to think about.  Oral Prednisolone in preschool children with virus-associated wheeze: a prospective, randomised, double-blind, placebo-controlled trial. S.J. Foster, M N Cooper, S Oosterhof, M L Borland The Lancet Respiratory Medicine 2018 January 17 https://doi.org/10.1016/S2213-2600(18)30008-0 Study Aim To assess the efficacy of oral prednisolone in children presenting to a paediatric emergency department with suspected viral wheeze. The study was originally a non-inferiority trial designed to test the hypothesis that a placebo is non-inferior to prednisolone, the authors added in a superiority analysis, testing the hypothesis that prednisolone was superior to placebo. The secondary hypothesis was added after the data had been collected but prior to data analysis. Participants Eligible patients were between 2- 6 years of age who presented to the paediatric emergency department in Princess Margaret Hospital, Perth, Australia and had a clinical diagnosis of wheeze combined with symptoms of a viral upper respiratory tract infection. Patients were excluded with: Oxygen saturations <92% in air, a silent chest, shock or sepsis, previous PICU admission with wheeze, prematurity, other cardio-respiratory disease, likely alternative diagnosis for wheeze, steroid treatment within the preceding 14 days, allergy to prednisolone or previous study recruitment. The eligibility criteria were suitable for the objective and looked at a population of children who regularly present with viral wheeze. The authors have excluded the bronchiolitis age group of children by excluding children under 2. This is important because they respond to different management. Design There were 3727 patients assessed for eligibility. After exclusions 312 were put into the placebo arm and 312 into the prednisolone arm. Following withdrawals 300 were included for analysis in the placebo group and 305 for the prednisolone group. Prior to data collection the authors calculated a sample size in order to ensure significant results. This was calculated on the original aim of non-inferiority. The study was a randomised double blind trial. Once eligible the participants were randomly assigned on a 1:1 basis to receive either 1mg/kg once daily of prednisolone or placebo. The placebo was formulated by the hospital and matched the prednisolone for volume, concentration, colour, smell and taste. The drugs were in matching bottles and randomisation was appropriate. The blinding for parents, patients, doctors, the research team and biostatician was kept until the statistical analysis was completed. The initial drug dose was administered by a nurse but for the following 2 days were given by the parents at home. The severity of the wheeze was assessed prior to treatment using a calculated pulmonary score. The family completed a questionnaire about home management and previous symptoms and a viral swab was taken from each participant. Each patient was managed with bronchodilators in line with the hospital policy. If the patient who was included in the study was admitted to the ward, and the admitting doctor felt that the patient should receive steroids, then these were given. 23 patients in the placebo group were given steroids in these circumstances. These patients were included in the intention to treat arm and the continuation of the study drug, alongside the prescribed prednisolone was at the discretion of the patient and their parents. They were included in the analysis and if anything would have made it more difficult to demonstrate a statistical significance between the groups. A pulmonary symptom scoring system was used for each patient to group wheeze severities together for analysis which enabled comparisons between groups, and to assess whether initial symptom severity was responsible for patient outcomes. All staff were competent in its use, however, this has score not been validated in children less than 5 years old. Outcomes This paper only comments on some of the data collected during the trial, the remaining data on long term outcomes will be presented in further manuscripts. The initial dual primary outcomes were length of stay in the emergency department and the total length of stay within the hospital. Secondary outcomes (within the first 7 days after hospital discharge) included: reattendance, readmission, salbutamol usage and residual symptoms after discharge. During the data collection it was felt the initial primary outcome; length of stay within the emergency department, was not reflective of the clinical condition as it was dependent on many non clinical factors. The study therefore only looked at an outcome of total length of stay in hospital until the participant was fit for discharge. This change was clearly outlined by the authors and appropriate for the aim of the study. Analysis The study groups were well balanced and the authors found no significant differences between groups in: baseline demographics; pulmonary score at presentation; personal or family history of atopy; use of salbutamol before attendance to the department. Categorical variables were analysed using a χ2 test or fishers exact test and continuous variables were compared using Students t test. Therefore a previously identified risk factor of personal or family history of atopy did not affect the outcomes in this study. Linear regression is the main analysis used to see if outcomes differ for the 2 groups. The authors conclude:
Subgroup analyses:
Secondary outcomes assessed were around representation post discharge. 26 patients re-attended (15 from prednisolone arm and 13 from the placebo arm). 3 patients from the prednisolone group and 2 patients from the placebo group were then prescribed steroids. One patient, from the prednisolone group needed admission to PICU. The statistical analysis of the reattenders (secondary outcomes) was not made clear within the article. Discussion The authors discuss their results in regards to the Panickar 2009 paper; they postulate that the study design could go some way to explaining the different outcome. The authors outline that they have tried to address some of the identified limitations found in the 2009 paper. These include the exclusion of children under 2 years old, thus reducing the likelihood for inclusion of patients with bronchiolitis. They urge some caution within their subgroup analysis conclusions as the sample size was smaller than the sample size used for calculating the general conclusion, also the patients were not randomly allocated to the arms based on the severity of symptoms or the use of salbutamol prior to attendance. This presentation is something we see daily in the emergency department and children’s assessment unit. It is an area for which I don’t feel that we have fully understood all the physiology behind the presentations. There are most likely many phenotypes of childhood wheeze. More research is definitely needed in order to assess the need for steroids and how to select the patients who would benefit from them. I look forward to reading further papers on the more long term conclusions of this trial. I think that I will still continue to judge the need for steroids on a patient by patient basis. Questioning with each patient whether they should or should not receive steroids. (And we’ve not even touched on Dexamethasone vs. Prednisolone!) It would be great to hear your views on this topic, I encourage you to comment on the blog or tweet me. (@RCBroomfield) Have a lovely February Rebecca The previous paper referenced can be found at: Panickar J, Lakhanpaul M, Lambert PC, Kenia P, Stephenson T, Smyth A, Grigg J. Oral Prednisolone for preschool children with acute virus induced wheezing, New England Journal of Medicine. 2009 Jan 22;360(4);329-38 |
Editors
Dr Annabel Greenwood Categories
All
|













 RSS Feed
RSS Feed
