|
Dr Tom Cromarty Editor Interests: Paediatric Emergency Medicine, Medical Engagement and Leadership, Simulation, Quality Improvement, Research Twitter: @Tomcromarty |
Welsh Research and Education Network
WREN BlogHot topics in research and medical education, in Wales and beyond
Dr Celyn Kenny Editor Interests: Neonates, Neurodevelopment, Sepsis, Media and Broadcasting Twitter: @Celynkenny |
|
‘Eculizumab in Shiga-Toxin producing E. Coli Haemolytic Uraemic Syndrome: A Randomised, Double-Blind, Placebo-Controlled Trial’ This is a trial currently recruiting on the Paediatric Nephrology Unit at UHW Aim of trial: To assess whether Eculizumab reduces the severity of Shiga-toxin producing Ecoli Haemolytic Uraemic Syndrome (STEC HUS) in children and young people Background:
What is Eculizumab?
Primary Research Objective: To determine whether the severity of STEC HUS is less in those given Ecu compared with those given placebo, in children aged 6m-18 years -Clinical Severity Score assigned at day 60 Secondary Objectives: i)To assess the safety of Ecu in STEC HUS ii)To determine whether the incidence of CKD following STEC HUS is less in those receiving Ecu compared with those receiving placebo iii)To evaluate the cost-effectiveness of administration of Ecu in STEC HUS from the perspective of the NHS Inclusion Criteria
- MAHA - Thrombocytopaenia - AKI
Exclusion Criteria
Active arm: Standard therapy + 1st dose Ecu Day 1, & 2nd dose Ecu Day 8 Control arm: Standard therapy + 1st dose placebo Day 1, & 2nd dose placebo Day 8 NB: The trial contains an internal pilot phase of 18m (12m recruitment, 6m follow-up), the purpose of which is to determine whether the substantive trial will continue. For further information please contact: Jennifer Muller, Paediatric Research Specialist Nurse (CYARU) [email protected] Annabel Greenwood
Paediatric ST3
1 Comment
By Annabel Greenwood The Neonatal Unit at UHW are currently participating in the ‘Optimist-A Trial’ and I thought this would be the perfect platform to raise awareness and explain briefly about the trial…
What is it? An international multicentre randomised controlled trial of surfactant administration whilst on CPAP, in preterm infants 25-28/40 gestation. Research Question Does administration of exogenous surfactant using a minimally-invasive technique improve outcome in preterm infants treated with continuous positive airway pressure (CPAP)? Why is it important? Premature babies ( ≤28 weeks gestation) with significant surfactant deficiency on CPAP have historically required intubation for the administration of surfactant therapy, with subsequent increased associated risk of death, bronchopulmonary dysplasia (BPD) and other morbidities. In an attempt to address this problem, minimally-invasive surfactant therapy (MIST) techniques have been developed whereby exogenous surfactant is administered via an instillation catheter, inserted briefly into the trachea under direct laryngoscopy, whilst on CPAP. Eligibility Criteria
Randomisation Eligible infants will be randomly allocated to receive exogenous surfactant via MIST, or continue on CPAP. Intervention
Primary Outcome
Any further queries? Contact Rachel Hayward Neonatal SpR at UHW, for any further information ([email protected])
Once randomized, children will be followed up over a 28-day period, with telephone contact at 7, 14 and 21 days, and a face-to-face consultation at 28 days. Parents are also keeping a daily symptom diary to monitor their child’s progress, as well as filling in wellbeing questionnaires at the contact times. Pre- and post-treatment nasoparyngeal and saliva swabs are being taken to analyse bacterial resistance patterns.
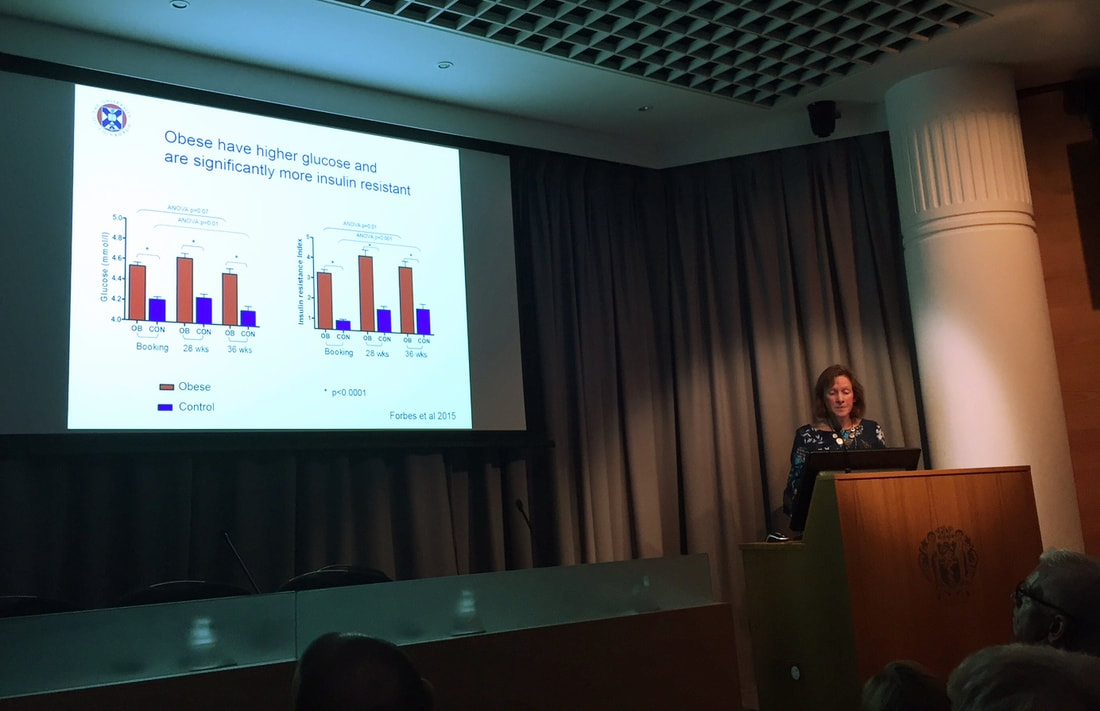
CAP-IT is planned to be recruiting over the next two winters with a target cohort of around 2,500 children, so is still in its early phases. It’s one of the first major trials being run out of the new Children and Young People’s Research Unit, based at the Noah’s Ark Children’s Hospital for Wales. For anyone that is interested and wants to know more, or even get involved, please get in touch with WREN and we can point you in the right direction! Links to the protocol and website are included at the bottom of the article. So, hopefully in a few years, we may understand this common condition a little better, but more importantly, the best way to treat it and ensure that treatment will continue working for the generations to come. CAP-IT: Efficacy, safety and impact on antimicrobial resistance of duration and dose of amoxicillin treatment for young children with Community-Acquired Pneumonia (CAP): A randomized controlled trial. www.capitstudy.org.uk http://www.ctu.mrc.ac.uk/our_research/research_areas/other_conditions/studies/cap_it/ 9th November 2017, Royal Society of Medicine, London By Chris Course On 9th November, the Neonatal Society held their Autumn Meeting at the Royal Society of Medicine in London. The program included oral presentations of submitted abstracts as well as invited guest speakers. There were a variety of topics covered, from studies looking at postnatal dexamethasone use upregulating surfactant proteins in preterm lamb models, surgical outcomes of preterm infants with congenital diaphragmatic hernia over a twenty-year period, increasing demands on neonatal unit for management of hypoglycaemia and breastmilk exposure influencing preterm brain development. The Widdowson lecture was given by Professor Pierre Gressens of King’s College and concerned the genomics of preterm brain injury. To be honest, some of the technicalities of the science and molecular pathways went a bit over my head, but there seemed to be some promising preliminary results on upregulating pro-repair inflammatory pathways and suppressing the damaging and immunomodulatory pathways by using tiny engineered pieces of DNA to protect the preterm brain’s white matter. Might seem a bit removed from clinical practice now, and seemed to be still a way from trials in human subjects, but then again, in a decade who knows what we’ll be doing on the wards! The keynote lecture came from Professor Rebecca Reynolds of Edinburgh University, who gave a fascinating talk on maternal health determining offspring life-course outcome. She went through data showing that it had been identified in the 1960’s that areas with high infant mortality ratios at the turn of the last century, went on to become areas with high rates of cardiovascular deaths in later life. Over the years, various studies have been conducted looking at the fetal programming effects of raised cortisol (secondary to maternal stress) reprogramming the fetal hypothalamo-pituitary-adrenal axis, and raised BMI/poor maternal diet/lifestyle leading to impaired insulin sensitivity in the fetus. Cohorts have been followed-up which show these infants have higher rates of type 2 diabetes and poorer neurodevelopmental outcomes/behavioural problems. There’s also a cohort of mothers in Finland that’s been followed up based on the amount of liquorice they consume…. Might seem odd, but apparently, it’s a popular snack in Finland, but suppresses an enzyme in the placenta that prevents too much cortisol passing into the fetus. It really highlighted how what we do in early life, and even before conception, can affect us for the rest of our lives! It did however also make me aware that in our role as paediatricians/neonatologists we have an opportunity to change long-term health for the better too. For anyone who hasn’t been to the Royal Society of Medicine, it’s a suitably impressive venue (befitting the name really) just behind Oxford Street, which was perfect for a leisurely lunch and a spot of early Christmas shopping during the society’s business meeting. And the day was rounded off with a drinks reception – what’s not to love! The day really brought home how diverse the neonatal research world is, and how much work is going on to try and improve understanding of physiology, diseases and develop new treatments. The Neonatal Society hold three meetings a year. The Autumn and Spring meetings are one-day, held in London, are free to attend and don’t require prior registration. The Summer meeting changes location around the UK (and sometimes ventures into Europe), with next year’s being held in Dublin in June. Abstracts are invited to be submitted for all the meetings, and are a great opportunity to get projects presented at a high-quality, national meeting – good for the CV/portfolio! Additionally, once you have presented at a society meeting, you are eligible to apply for membership.
For more information, check out www.neonatalsociety.ac.uk St Brides Hotel, Saundersfoot, Friday 10th November 2017 Hosted by Glangwili General Hospital & Bronglais Hospital By Annabel Greenwood This Autumn’s WPS meeting took place in the absolutely stunning location of St Brides Hotel in the picturesque seaside town Saundersfoot. Nestled in the clifftop, overlooking the breath-taking rugged Welsh coastline, it provided the perfect backdrop for an invigorating day of presentations, discussions and networking. The day began with the invitation lecture from the esteemed Dr Trevor Brown, Consultant Paediatric Allergist at Ulster Hospital, on cow’s milk allergy (formerly cow’s milk protein allergy). Ever a diagnostic and management paradox for GPs and general paediatricians, this in-depth discussion covering IgE verus non-IgE mediated reactions, clinical presentation and potential management options was invaluable. The morning session presentations were abundant in variety and interest, including projects and audits from Cardiff University Medical Students, Welsh Paediatric trainees, and Consultants. Of highlight during this session was the fantastic prize-winning project presented by our very own WREN co-chair Dr Siwan Lloyd on head injury following infant falls on the postnatal ward – a case series. The variety in the approach to investigation and management of such patients was fascinating, especially given the high rate of CT head abnormalities of those imaged. The subsequent departmental guideline implemented at UHW will certainly help standardise care and provide more clarity and direction for management. Almost time for lunch, but of course not before the brilliant Guest Lecture from Detective Superintendent Anthony Griffiths, Head of Public Protection, Dyfed Powys Police – ‘Child Protection: The Challenges for Policy and Multi-Agency Working.’ This lecture provided an invaluable insight into the challenges often faced by the police in child protection cases. The videos and images used were extremely powerful and emphasized the great difficulties in tracking down offenders in today’s society, particularly given the huge smart phone and social media influence. After an action-packed morning of presentations and lectures it was time to refuel with a mouth-watering lunch overlooking the absolutely spectacular scenery. Coffee could be taken onto the balcony, and what a treat it was to catch up with friends and colleagues with the sound of the waves crashing onto the shore below. There was also the opportunity during this lunchbreak to visit the exhibitor stands, the main theme being cow’s milk allergy, in-keeping with the invitation lecture earlier in the day, and there were a variety of different formulas to sample at these stands (some of which far more palatable than others!!) The afternoon session was equally interesting and stimulating, including presentations inspiring and empowering trainees, highlighting the importance of blogging, social media and trainee-led initiatives to encourage learning and champion change. The final Guest Lecture of the day was provided by the highly-regarded Dr Julian Forton, Consultant in Paediatric Respiratory Medicine and Cystic Fibrosis, UHW, on ‘the new C21 Curriculum at Cardiff University School of Medicine.’ It was absolutely fascinating to learn how the curriculum and course structure has evolved since my days in medical school! The aim is for a more case-based, small group learning approach with early clinical exposure. The exam format was also an interesting change, whereby all years take the same end-of-year exam, with the focus being on demonstrating improvement and development each year. As the sun was setting at the end of a wonderful day, it was time for the evening entertainment in the form of the ‘celebrating trainee event’ followed by the formal evening dinner. This year the Best Trainee prize went to Dr Siwan Lloyd in South Wales (taking a clean sweep of prizes!) and to Dr Stacey Killick in the North. Best Educational Supervisor went to Dr Zoe Roberts…congratulations to all!
The day was wrapped up with a splendid evening of dinner and dancing (thank goodness for the spa to recover the next morning!) Once again, WPS has delivered an outstanding conference! It provides an excellent platform to showcase your work, and to become inspired by colleagues and friends. It also provides the opportunity to learn from lead professionals in their specialised field, and to celebrate the achievements of others. We certainly are lucky to have such a wonderful paediatric society in Wales! Now, who’s joining me for the Spring meeting?!.... |
Editors
Dr Annabel Greenwood Categories
All
|

















 RSS Feed
RSS Feed
